Week 3: Volumetric Behaviour of (binary) Mixtures from Intermolecular Interactions#
In the case of mixtures of monoatomic, spherical molecules, the second order virial coefficient can be computed as:
\[
B_{mix}=y_iB_{ii}+y_jB_{jj}+2y_iy_jB_{ij}
\]
where \(B_{ij}\) is:
\[
B_{ij}=2\pi{N_A}\int_0^\infty((1-\exp(-\Gamma_{ij}/RT))r^2)dr)
\]
This requires obtaining an interaction potential \(\Gamma_{ij}\) for \(i\neq{j}\), it can be done via mixing rules.
Binary Mixture - System Definition#
import matplotlib.pyplot as plt
from matplotlib import cm
import numpy as np
from mpl_toolkits.mplot3d import Axes3D
#Species=[Ar, Kr, Xe, CH4, N2, C2H4, C2H6, C3H8]
SIGMA=[3.499, 3.846, 4.100, 4.010, 3.694, 4.433, 5.220, 5.711] # Angstrom
EPS=[118.13, 162.74, 222.32, 142.87, 96.26, 202.52, 194.14, 233.28] #K
#Specie 1 and 2
S1=3
S2=6
#Parameters for the Pure Components
SIGMA1=SIGMA[S1];
SIGMA2=SIGMA[S2];
EPS1=EPS[S1];
EPS2=EPS[S2];
#Lorentz Berthelot Mixing Rules
SIGMA12=np.mean((SIGMA1,SIGMA2))
EPS12=np.sqrt(np.prod((EPS1,EPS2)))
# Temperature range
Temperature = np.linspace(300, 500, 10)
# Gas constant
R=0.082; # l atm K^-1 mol^-1
---------------------------------------------------------------------------
ModuleNotFoundError Traceback (most recent call last)
Cell In[1], line 1
----> 1 import matplotlib.pyplot as plt
2 from matplotlib import cm
3 import numpy as np
ModuleNotFoundError: No module named 'matplotlib'
Two body interaction potentials#
SIGMA_mixture=np.array((SIGMA1,SIGMA2,SIGMA12))
EPS_mixture=np.array((EPS1,EPS2,EPS12))
figure=plt.figure()
axes = figure.add_axes([0.1,0.1,1.5,1.5])
plt.xticks(fontsize=14)
plt.yticks(fontsize=14)
axes.set_xlabel('r, A', fontsize=14);
axes.set_ylabel('$\Gamma$, [K]',fontsize=14);
axes.set_xlim([0.5,20]);
axes.set_ylim([-250,50]);
r = np.linspace(0.3, 25, 500)
Gamma=np.zeros((np.size(r),np.size(SIGMA_mixture)))
color=iter(cm.gist_heat(np.linspace(0,1,np.size(SIGMA_mixture)+1)))
for i in np.arange(0,np.size(SIGMA_mixture)):
c=next(color)
sr6=np.power(np.divide(SIGMA_mixture[i],r),6)
Gamma[:,i]=4*EPS_mixture[i]*(np.power(sr6,2)-sr6)
axes.plot(r,Gamma[:,i], marker=' ' , c=c);
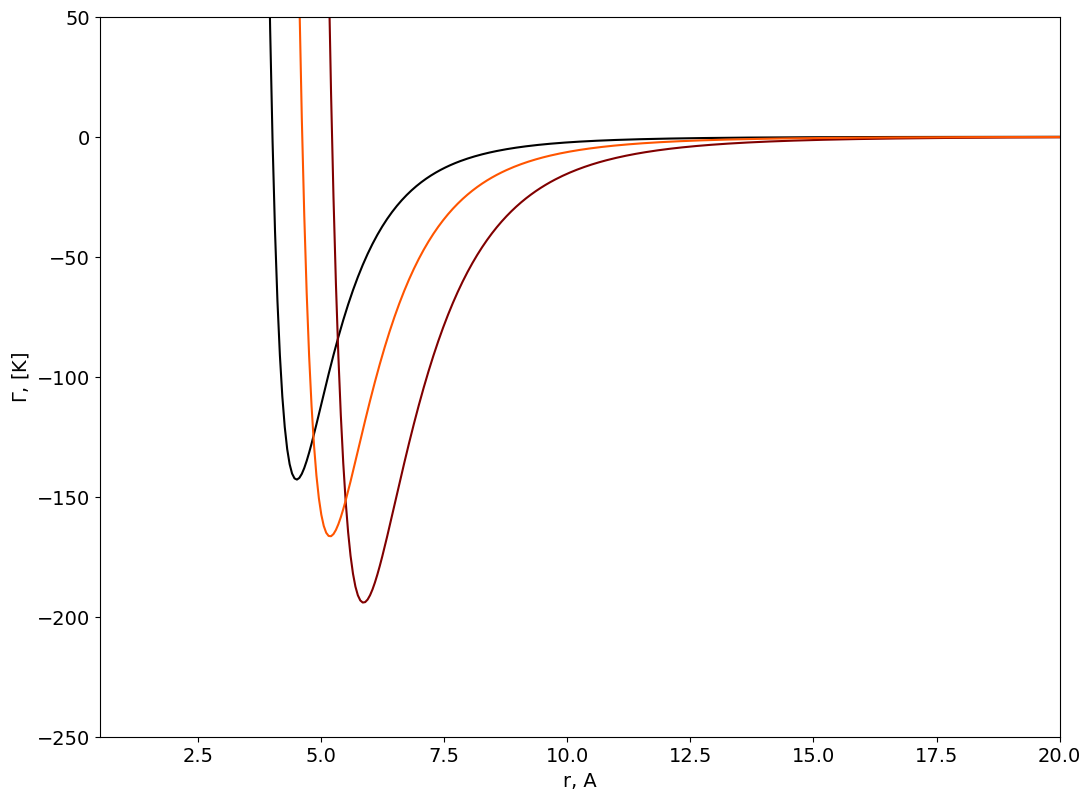
Second Virial Coefficient#
# Compute the second Virial coefficient as a function of T
figure=plt.figure()
axes = figure.add_axes([0.1,0.1,1.5,1.5])
plt.xticks(fontsize=14)
plt.yticks(fontsize=14)
axes.set_xlabel('Temperature, [K]', fontsize=14);
axes.set_ylabel('B, dm$^3$ mol$^{-1}$',fontsize=14);
B=np.zeros((np.size(Temperature),np.size(SIGMA_mixture)))
color=iter(cm.gist_heat(np.linspace(0,1,np.size(SIGMA_mixture)+1)))
for i in np.arange(0,np.size(SIGMA_mixture)):
c=next(color)
for j in np.arange(0,np.size(Temperature)):
B[j,i]=2*np.pi*6E23*np.trapz((1-np.exp(-Gamma[:,i]/Temperature[j]))*np.power(r*1E-9,2),r*1E-9)
axes.plot(Temperature,B[:,i], marker=' ' , c=c);
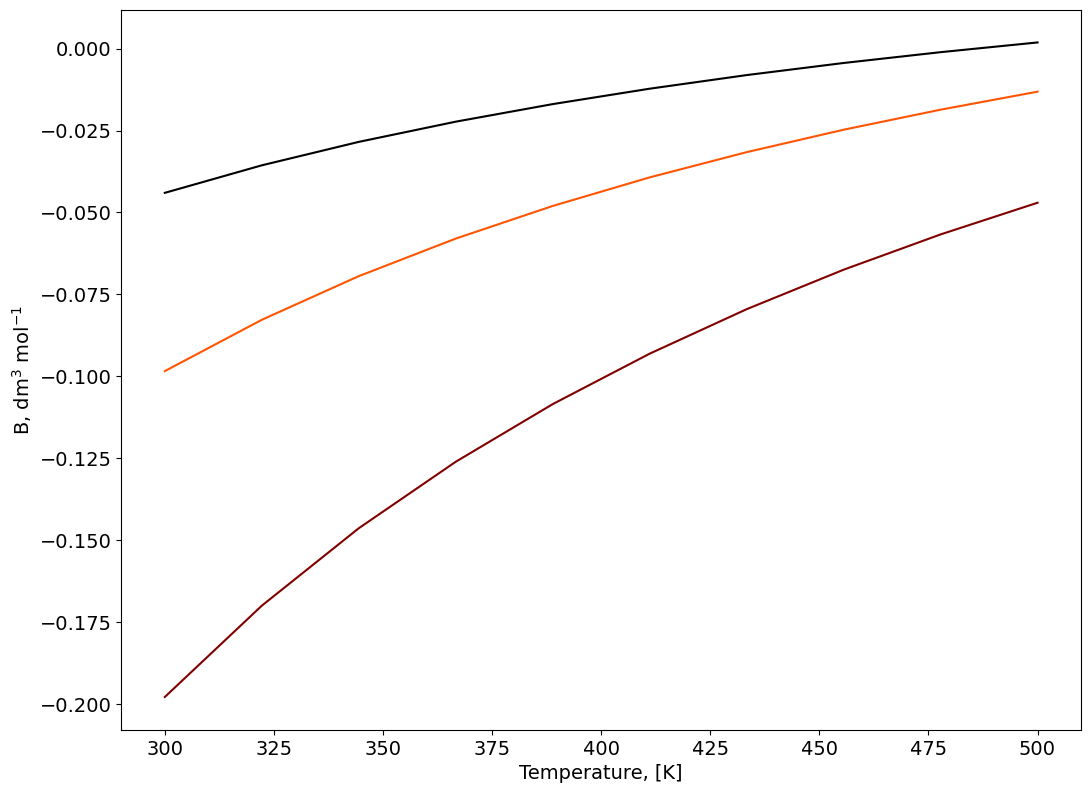
Second Virial Coefficient, function of the mixture composition.#
y1=np.linspace(0, 1, 20) # [-]
y2=1-y1
Y1, T = np.meshgrid(y1, Temperature)
Bmix=np.zeros((np.size(Temperature),np.size(y1)))
figure=plt.figure(figsize=(12, 10))
axes = figure.add_subplot(projection ='3d')
for i in np.arange(0,np.size(Temperature)):
Bmix[i,:]=B[i,0]*Y1[i,:]*Y1[i,:]+B[i,1]*(1-Y1[i,:])*(1-Y1[i,:])+B[i,2]*Y1[i,:]*(1-Y1[i,:])
surf=axes.plot_surface(Y1,T,Bmix,cmap=cm.coolwarm,
linewidth=0, antialiased=False,alpha=0.5)
axes.view_init(25, -60)
plt.draw()
axes.set_xlabel('$y$', fontsize=14);
axes.set_ylabel('$Temperature, (K)$',fontsize=14);
axes.set_zlabel('B$_{mix}$, dm$^3$ mol$^{-1}$',fontsize=14);
figure.colorbar(surf, shrink=0.5, aspect=10);
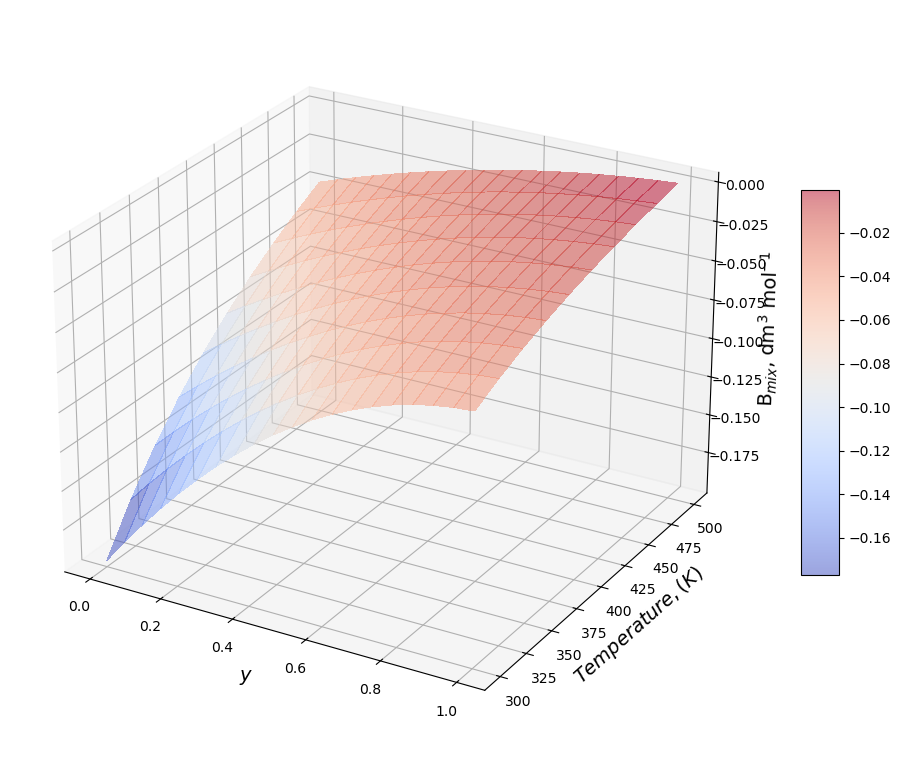
Properties#
Compressibility factor at constant \(\rho_{mix}\)#
## Define the total density of the mixture
rho=10 # mol / l
## Z Diagram
Z=1+rho*Bmix
figure=plt.figure(figsize=(10, 8))
axes = figure.add_subplot(projection ='3d')
axes.view_init(25, -55)
plt.draw()
surf=axes.plot_surface(Y1,T,Z,cmap=cm.coolwarm,
linewidth=0, antialiased=False,alpha=0.5)
axes.set_xlabel('$y$', fontsize=14);
axes.set_ylabel('$Temperature, (K)$',fontsize=14);
axes.set_zlabel('B$_{mix}$, dm$^3$ mol$^{-1}$',fontsize=14);
figure.colorbar(surf, shrink=0.5, aspect=10);
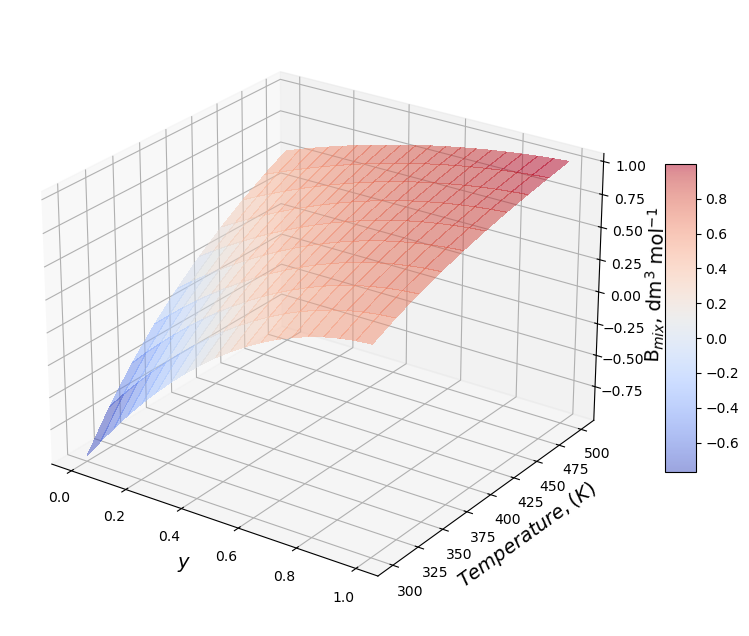
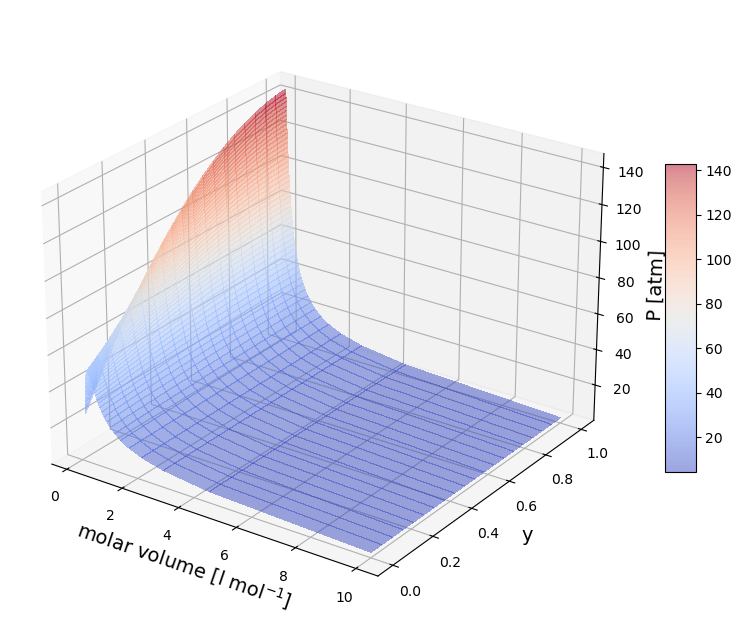
P/v/y diagram at constant T#
## Define the Temperature
T=350;
rho=np.linspace(0.1, 6, 50) # mol / l
rho_g, Y2 = np.meshgrid(rho, y1)
BT=np.zeros(np.size(SIGMA_mixture))
for i in np.arange(0,np.size(SIGMA_mixture)):
BT[i]=2*np.pi*6E23*np.trapz((1-np.exp(-Gamma[:,i]/T))*np.power(r*1E-9,2),r*1E-9)
BmixT=BT[0]*Y2*Y2+BT[1]*(1-Y2)*(1-Y2)+BT[2]*Y2*(1-Y2)
P=T*R*rho_g+T*R*rho_g*rho_g*BmixT
Z=1+rho_g*BmixT
figure=plt.figure(figsize=(10, 8))
axes = figure.add_subplot(projection ='3d')
axes.view_init(25, -55)
plt.draw()
surf=axes.plot_surface(1/rho_g,Y2,P,cmap=cm.coolwarm,
linewidth=0, antialiased=False,alpha=0.5)
axes.set_xlabel('molar volume [l mol$^{-1}$]', fontsize=14);
axes.set_ylabel('y',fontsize=14);
axes.set_zlabel('P [atm]',fontsize=14);
figure.colorbar(surf, shrink=0.5, aspect=10);
figure=plt.figure(figsize=(10, 8))
axes = figure.gca(projection ='3d')
axes.view_init(25, -55)
plt.draw()
surf=axes.plot_surface(1/rho_g,Y2,Z,cmap=cm.coolwarm,
linewidth=0, antialiased=False,alpha=0.5)
axes.set_xlabel('molar volume [l mol$^{-1}$]', fontsize=14);
axes.set_ylabel('y',fontsize=14);
axes.set_zlabel('compressibility factor',fontsize=14);
figure.colorbar(surf, shrink=0.5, aspect=10);
---------------------------------------------------------------------------
TypeError Traceback (most recent call last)
Cell In[8], line 33
30 figure.colorbar(surf, shrink=0.5, aspect=10);
32 figure=plt.figure(figsize=(10, 8))
---> 33 axes = figure.gca(projection ='3d')
34 axes.view_init(25, -55)
35 plt.draw()
TypeError: FigureBase.gca() got an unexpected keyword argument 'projection'
<Figure size 1000x800 with 0 Axes>
